Poxviridae
http://www.sciencedaily.com/images/2007/04/070430155718-large.jpg
The poxviruses, another group of enveloped, linear dsDNA viruses, are the largest and most complex of all viruses. There are terminal repeated sequences and inverted repeats at both ends. They are widely distributed in nature. Nearly every animal species can be infected by a form of poxvirus. The human poxviruses (orthopoxviruses) are large, enveloped, brick-shaped viruses. These viruses multiply in specialized portions of the host cell cytoplasm called viroplasm, where they can cause skin lesions typical of smallpox and coxpox. Other poxviruses, such as monkeypox, can infect humans who have close contact with infected animals.
Important properties
· Large family of viruses
· Large enough just to be seen under a light microscope.
· Unique oval-shaped
· Antigenically very complex
· Unlike the influenza virus, poxviruses can remain stable for hours in the air. It could be due to the fact that it is an enveloped virus.
Smallpox virus is an example of a poxvirus.
Transmission
· Human is the only reservoir for smallpox
· Respiratory secretions (aerosolized air droplets) and saliva and bodily fluids from infected person are sources of transmission
Clinical Features
· ~12 days incubation
· Initially influenza like symptoms
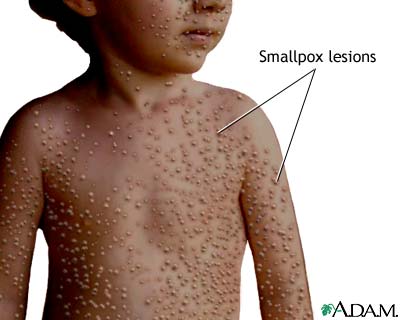
· Characteristic pustules
· Always associated with skin lesions and scarring of skins
· Neurological damage
· Blindness
· Death
http://www.nlm.nih.gov/medlineplus/ency/images/ency/fullsize/19059.jpg
Two varieties of smallpox are:
1 Variola Major
a. 25%-30% fatalities.
2 Variola Minor
a. Less than 1% death
Vaccination
In 1796, Edward Jenner developed a smallpox vaccine using cowpox. He used cowpox from milkmaids and inoculated it into an 8 year old boy James Phipps. When challenged with smallpox, he recovered quickly and gained immunity to smallpox.
Eradication of Smallpox
· The variola virus has no reservoir except man
· It only has a single, stable serotype, which is the key success of vaccination.
· It causes only acute infection from which the patient dies, or
· Obtains lifelong immunity to it as
· Variola virus is an effective immunogen (sure to gain immunity from it)
· Vaccination programs
· Certified by WHO in 1980 to be defeated and eradicated off the face of the earth.
Laboratory Diagnosis
· Diagnosis was made either by growing the virus in cell culture or chick embryos or by detecting viral antigens in vesicular fluid by immunofluorescence.
Control
· Vaccination
· Incineration of all infected material.
· However, vaccination no longer required after 1980, when smallpox was eradicated.
· Smallpox remains as one of the deadliest virus around—it is still kept in certain laboratories. There have been concerns that smallpox may be used in biowarfare and bioterrorism.