In the 1920s, several cases of a slow but progressively dementing illness in humans were observed independently by Hans Gerhard Creutzfedlt and Alfons Maria-Jakob. It was characterized by mental degeneration, loss of motor function, and eventual death.
In 1982, Stanley Prusiner proposed that the infective causative agent is an exceedingly small proteinaceous infectious particle called a prion.
Introducing Prions
Prions are rogue proteins that transform other cellular protein (PrPC) to the prion form PrPSC. Research indicates that prions are normal proteins that become folded incorrectly (possibly due to mutations).
Prions cause fatal, neurological degenerative diseases, including Creutzfeldt-Jakob disease (CJD), kuru, scrapie (Transmissible spongiform encephalopathy), mad cow disease (Bovine spongiform encephalopathy) and chronic wasting disease. The disease can be passed on to humans—new-variant CJD.
Important Characteristics
· Prions are resistant to inactivation by heating to 90°C, which will inactivate viruses.
· Prion infection is not sensitive to radiation treatment that damages virus genomes.
· Prions are not destroyed by enzymes that digest DNA or RNA.
· Prions are sensitive to protein denaturing agents such as phenol and urea.
· Prions have direct pairing of amino acids.
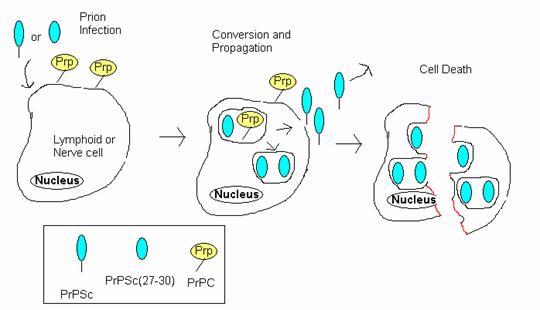
Mechanism of Action
http://www.bio.davidson.edu/Courses/Molbio/MolStudents/spring2005/Winter/clip_image012.jpg
1. Initial infection and eventual interaction with normal cellular PrPC.
a. PrPSc forms a heterodimer with normal PrPC
b. Template for altering the protein fold
c. Tightly coiled alpha helix becomes converted to loose beta sheets.
2. PrPC and PrPSc are internalized and interact within caveolae-like vesicles where PrPC is converted to PrPSc.
3. PrPSc may be recycled to the cell surface(shedding) to interact with PrPC from other cells and spread the infection, or remain and accumulate within the cell.
4. When PrPSc accumulates in the cell, they stick together inside cells, forming small fibrils. Because the fibrils cannot be organized in the plasma membrane correctly, such aggregations eventually kill the cell.
5. Cell death would release PrPSc and spread the infection; partially released proteolyzed PrPSc 27-30 may aggregate within plaques.
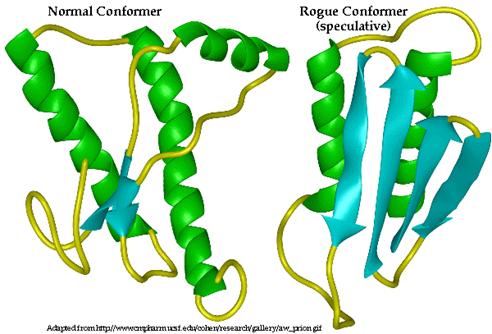
http://www.bio.davidson.edu/Courses/Molbio/MolStudents/spring2005/Winter/clip_image010.jpg
In Step 1(c): Three-dimensional structure of PrP C (left) and proposed 3D structure of PrP Sc (right). Alpha helices are indicated in green while beta sheets are indicated in blue. PrP C is composed primarily of alpha helices while PrP Sc is composed primarily of Beta pleated sheets.
Note:
· α-helical, cellular isoform of the prion protein is PrPC. It is a membrane-anchored glycoprotein.
· Insoluble, β-sheet, infectious, disease-causing isoform is PrPSc
| PrP C | PrP Sc |
| primarily beta sheet structure | |
| protease susceptible | resistant to protease |
| monomeric species | forms multimeric aggregates |
| stable monomer | less stable monomer, self propagates for stability |
| normal resistance | extremely resistant to heat, chemicals, radiation and strong solvents |
| detergent soluble | detergent insoluble |
| · Mouse with knockout PrPC gene · No PrPC protein · Infect with prions · Nothing to convert · Mouse lives | Mouse with normal PrPC gene Produce PrPC protein Infect with prions Prions convert normal PrPC to rogue proteins Mouse dies |
Pathogenesis
On the light-microscopic level spongiform changes in the brain, degeneration of neurons and astrocytosis are histopathological hallmarks of prion diseases.
Transmission:
Eating beef or mutton contaminated with prions.
Model of propagation of PrPSc
Viroids
· Viroids are infectious agents composed exclusively of a single piece of circular single stranded RNA which has some double-stranded regions.
· They mainly cause plant diseases (25 main sequences identified) by interfering with mRNA processing—they are catalytic ribozymes that cleave RNA to produce fragments containing a 5’-hydroxyl and a 2’, 3’-cyclic phosphate.
· Only one sequence is known to infect man (Hepatitis D)
Hepatitis D
· Only human disease known to be caused by a viroid.
· Previously called delta agent
· There is extensive sequence complementarity between the hepatitis D viroid RNA and human liver cell 7S RNA
o The 7S RNA structure involved in the translocation of secretory and membrane-associated particles.
· Hepatitis D viroid causes liver cell death via sequestering this 7S RNA and/or cleaving it.
Transmission
· Co-infection with Hepatitis B virus
· Bodily fluids
o Unprotected sex
o Sharing contaminated needles
o Close proximity
http://www.thailabonline.com/blood/hepdslide_1.gif
Virusoid
Virusoids are infectious agents that infect plants in conjunction with an assistant virus.
They are not considered viruses but are subviral particles.
The size and structure is similar to viroids.
Its genome is a single molecule of single stranded circular RNA that is several hundred nucleotides long and codes for nothing but its own structure.


0 comments:
Post a Comment